AARP Eye Center
Is Implantable Device Reform Almost Here?
By Keith Lind, December 21, 2018 11:04 AM
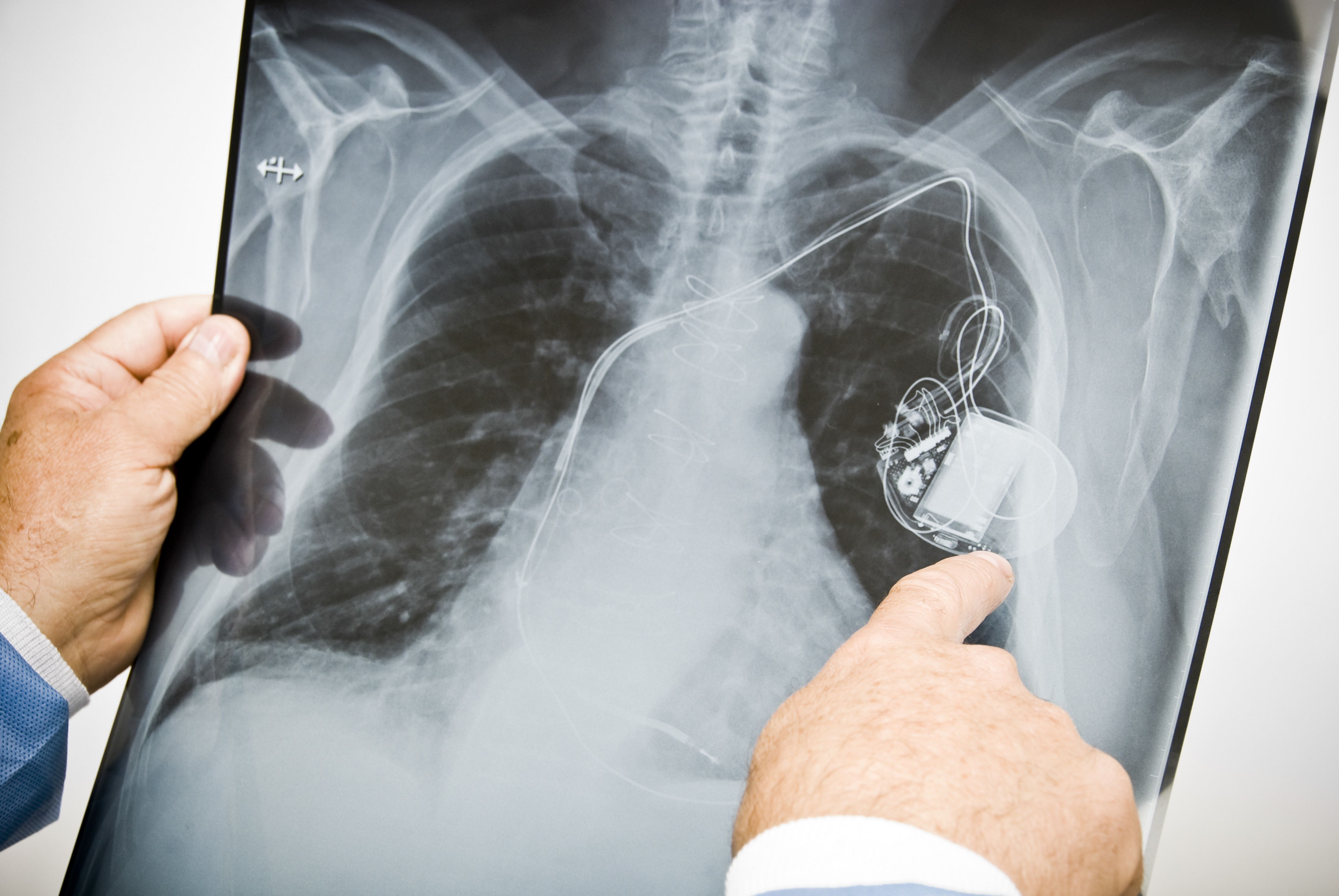
It’s not always an easy question for a consumer to answer, yet it comes up for more people than you might think. In the case of certain heart defibrillators, for example, consumers with defective devices run the risk that their device will either deliver an unnecessary jolt of electricity—akin to being hit across the chest with a baseball bat—or, worse, simply fail, potentially leading to cardiac arrest and death.
Unfortunately, more than a few implantable devices have become defective. An investigation by the International Consortium of Investigative Journalists (ICIJ) found that many governments around the world have allowed products onto the market after going through little or no human testing, and wound up causing great harm to consumers. In the U.S., the report found potential links between faulty medical devices and more than 1.7 million injuries and nearly 83,000 deaths over the last decade. One problem that the investigation highlighted is that manufacturers are allowed to “piggyback” approval for a new device onto previously marketed devices.
The ICIJ report also revealed that:
- the device industry and the regulators that oversee it struggle to quickly identify hazardous implants after they are released, leaving patients exposed
- devices pulled off the market in some countries over safety concerns remain for sale in others
Many of the ICIJ investigation findings mirror those in an AARP Public Policy Institute report, “Implantable Devices: Regulatory Framework and Reform Options,” which explored areas of public concern surrounding implantable devices.
Fortunately, reform may be on the way here in the U.S. The Food and Drug Administration (FDA) recently proposed to substantially alter the approval process for many medical devices. The revised approval pathway would be based on objective safety and performance criteria. The FDA would also limit the ability of manufacturers to gain approval of devices by piggybacking their approval on previously marketed devices.
The changes proposed by the FDA closely track with policy changes proposed in AARP’s implantable device paper. (That paper included many other policy options for consideration as well.)
Meanwhile, a note for consumers concerning the ICIJ report: In addition to serving the purpose of calling attention to the issue, the report is a useful resource. It includes a searchable list of device recalls and safety alerts—more than 70,000 from 11 countries—that anyone can access, allowing people to check whether a given device has been formally flagged over safety concerns.
We continue to advocate for open dialogue on issues related to implantable devices and for policy changes that both strengthen and streamline the process. Such changes will better protect public health and safety while also encouraging the development and marketing of devices that will benefit patients. We hope to provide positive updates on the FDA’s proposal.

Keith Lind is a senior strategic policy advisor at the AARP Public Policy Institute. His areas of expertise include Medicare, hospital observation, medical devices, and fraud and abuse.